Abstract
Background: Less than half of patients with lymphoma present with stage I or II disease and this presentation may be nodal or extranodal. Stage I lymphoma is commonly treated with chemotherapy with or without radiation therapy (RT), however little is known if primary site of involvement and treatment modalities affect survival outcome.
Patients and Methods: TheSurveillance, Epidemiology and End Results (SEER) database was used to evaluate survival outcomes among adult (≥18 yrs) patients diagnosed with stage I lymphoma between 1998 and 2014. We excluded histologic subtypes that develop in extranodal sites such as cutaneous T-cell lymphoma, enteropathy-associated T-cell lymphoma, hepatospenic T-cell lymphoma and extranodal NK-cell lymphoma. We also excluded central nervous system and testicular lymphoma. Overall survival (OS) was calculated from diagnosis to death from any cause using the Kaplan-Meier method. Differences in the survival function between groups were analyzed using the log-rank test. Cox proportional hazards models were used to evaluate associations between patient characteristics and survival.
Results: A total of 72095 patients were diagnosed with stage I disease during the study period. The median age of the patients was 66 years (range, 18-104 years). After excluding diseases as described above, diffuse large B-cell lymphoma (DLBCL) was the most common histology (N=24,574), followed by follicular lymphoma (FL, N=13,061), marginal zone lymphoma (MZL, N=9,893), Hodgkin lymphoma (HL, N=5,325) and peripheral T-cell lymphoma (PTCL, with PTCL not otherwise specified, anaplastic large cell lymphoma and angioimmunoblastic T-cell lymphoma, N=2,071). HL was excluded from the analysis with few extranodal diseases. With a median follow up of 69 months, the median OS was 104, 179, 165 and 106 months in patients with DLBCL, FL, MZL and PTCL, respectively. Thirty-seven percent of patients received resection of primary tumor. Thirty-nine percent of patients received neither RT (40% of them received surgical resection) nor chemotherapy, 15% of patients received RT alone, 29% of patients received chemotherapy alone and 17% of patients received combined modality therapy (CMT).
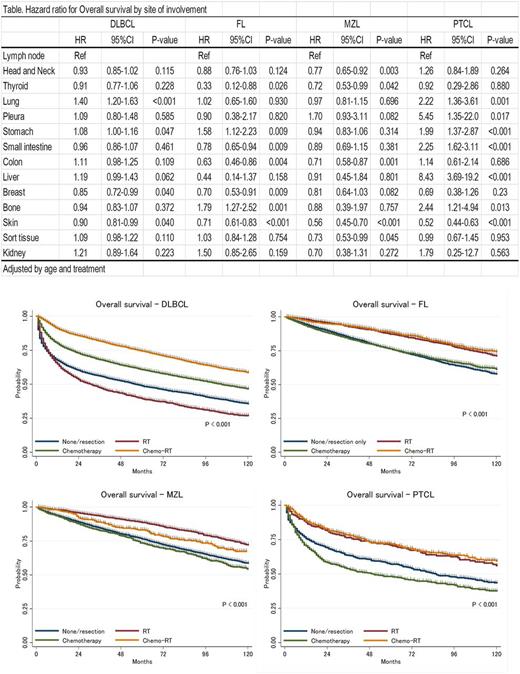
Hazard ratios (HR) for OS in stage I extranodal disease compared to stage I nodal disease in each histologic subtype are summarized in Table. Skin origin was associated with longer OS in all histologic subtypes. Breast origin was associated with longer survival in DLBCL and FL. Thyroid origin was associated with longer survival in FL and MZL. Stomach origin was associated with shorter survival in DLBCL, FL and PTCL but not in MZL. Small intestine origin was associated with longer survival in FL but associated with shorter survival in PTCL. Bone origin was associated with shorter survival in FL and PTCL. The difference in HR for site of involvement among histologic subtype is significant; overall, compared to nodal stage I disease, extranodal stage I disease was associated with longer survival in MZL but associated with shorter survival in PTCL.
CMT was significantly associated with longer survival in DLBCL while RT alone showed similar survival outcome in FL, MZL and PTCL (Figure). Local control by surgical resection was also associated with significantly longer survival in all histologic subtypes; HR for surgical resection was 0.81 (p<0.001) in DLBCL, 0.83 (p<0.001) in FL, 0.87 (p=0.002) in MZL and 0.85 (p=0.033) in PTCL.
Summary: There was a significant difference in survival outcome in stage I lymphoma by site of involvement. RT showed significant impact in survival outcome for patients with stage I FL, MZL and PTCL.
Fanale: AMGEN: Membership on an entity's Board of Directors or advisory committees; GENENTECH: Research Funding; MOLECULAR TEMPLATES: Research Funding; TAKEDA: Honoraria, Research Funding; SEATTLE GENETICS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MERCK: Membership on an entity's Board of Directors or advisory committees, Research Funding; ONYX: Research Funding; ADC THERAPEUTICS: Research Funding; CELGENE: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Westin: Apotex: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals Corporation: Membership on an entity's Board of Directors or advisory committees. Nastoupil: Abbvie: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Karus Therapeutics: Research Funding; TG Therapeutics: Honoraria, Research Funding; Gilead: Honoraria. Neelapu: Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Poseida Therapeutics, Inc: Research Funding; Cellectis Inc.: Research Funding; Karus: Research Funding; Bristol-Myers Squibb: Research Funding; Kite Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal